


TECHNOLOGY - MicroED
B A C KStage-camera synchronization
for conventional CryoEM
We developed a stage-camera synchronization scheme and automatic data collection software-eTasED for conventional Cryo-EM, making it possible to solve small molecule of protein structures in ultrahigh-resolution using a low-end electron microscope and improving the general applicability of MicroED.
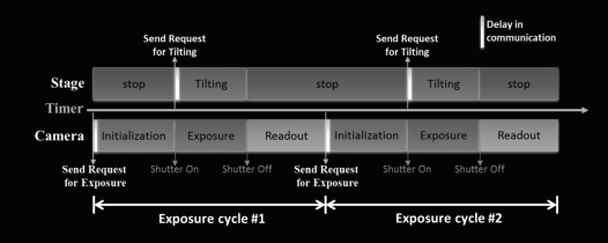
Fig1. Schematic diagram of the stage-camera synchronization.
MicroED are revealing structures at an increasing pace, though the technique is still undergoing development.
Growth of large and high-quality crystals represents the main bottleneck of structure determination by crystallography approaches. Traditional X-ray crystallography can not use crystals smaller than ~5 μm. As a novel crystallographic method, MicroED exploits the advantage that electrons interact much more strongly with material and posit considerably less damaging energy into a crystal. Electron diffraction data can be collected from extremely small nanocrystals at a low dose, which benefits structure research of crystallizing problematic proteins. With this new modality in cryo-EM, novel structures considered untraceable before were successfully determined today.

Figure 2. Crystal size difference between MicroED and X-ray crystallography.
However, special requirement for radiation-tolerated movie-mode camera and the lack of automated data collection method increases the barrier to the utilization of MicroED. ShuimoBio drives the development of useful software to simplify and speed workflows in a way that broadens accessibility to MicroED. We devised a stage-camera synchronization scheme eTasED to minimize the hardware requirements, which will accelerate the adoption of best practices for access.

Figure 2. Crystal size difference between MicroED and X-ray crystallography.
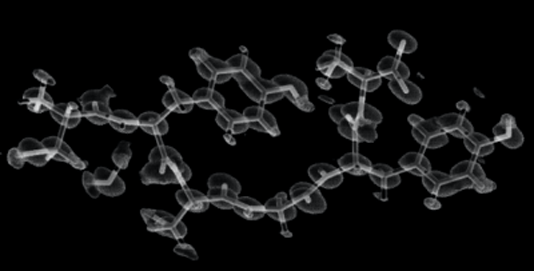
Figure 3. Ultrahigh-resolution structure constructed from the merged datasets collected by eTasED.
Benefiting from eTasED, microED can be realized on a conventional cryo-EM system without any modification
Published work using MicroED are still limited to several groups given to the requirement of specific camera hardware and high-end microscopes. Development of stage-camera synchronization scheme will advance the field and demystify more crystal structure.
- Tailored for single-frame camera. The stage tilting and sample illumination are activated and inactivated at predefined time points so that they are only activated within the effective exposure step of an exposure cycle.
- Available for movie-mode camera. With a movie-mode camera, the exposure time can be set much longer than that of a single-frame camera, such that each tilting-exposure cycle covers a large or even entire range of the tilting angle.
- Compatible with 120kV TEM. Ultrahigh-resolution diffraction data of peptide nanocrystals is collected on 120-kV electron microscopes, resulting in resolution up to ∼0.60 Å with unambiguous assignment of nearly all hydrogen atoms.
Featured Publication
The CryoEM Method MicroED as a Powerful Tool for Small Molecule Structure Determination
Jones CG et al. MicroED & Small molecular DOI:10.1021/acscentsci.8b00760
In the many scientific endeavors that are driven by organic chemistry, unambiguous identification of small molecules is of paramount importance. However, X-ray crystallography is rarely applied in routine organic chemistry due to intrinsic limitations of both the analytes and the technique. This paper demonstrates that MicroED can provide routine and unambiguous structural determination of small organic molecules. From simple powders, with minimal sample preparation, it could collect high-quality MicroED data from nanocrystals (∼100 nm, ∼10−15 g) resulting in atomic resolution (<1 Å) crystal structures in minutes.
V i e wStructure of the toxic core of α-synuclein from invisible crystals
Rodriguez J et al. MicroED & Protein DOI:10.1038/nature15368
The protein α-synuclein is the main component of Lewy bodies, the neuron-associated aggregates seen in Parkinson disease and other neurodegenerative pathologies. An 11-residue segment, which we term NACore, appears to be responsible for amyloid formation and cytotoxicity of human a-synuclein. As the crystals of NACore are thousands of times too small for structure determination by synchrotron X-ray diffraction, here used micro-electron diffraction to determine the structure at atomic resolution, providing certain clues for the in-depth understanding of various neurodegenerative diseases.
V i e w